bischler-mohlau吲哚合成反应亦称bischler吲哚合成反应。从α-卤代芳香酮和过量的苯胺环化生成3-芳基吲哚。
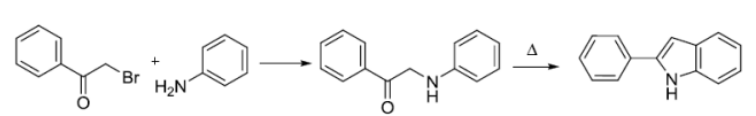
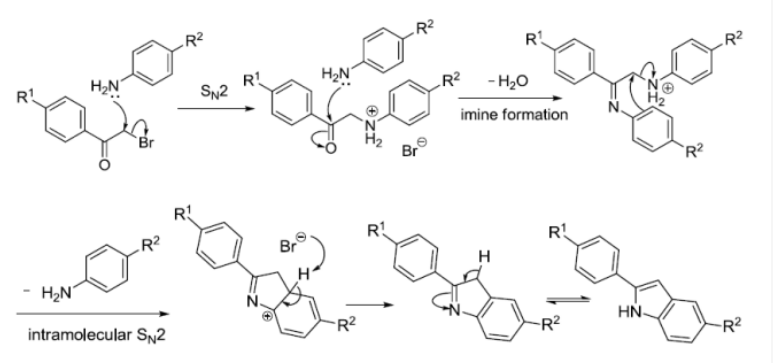
反应机理
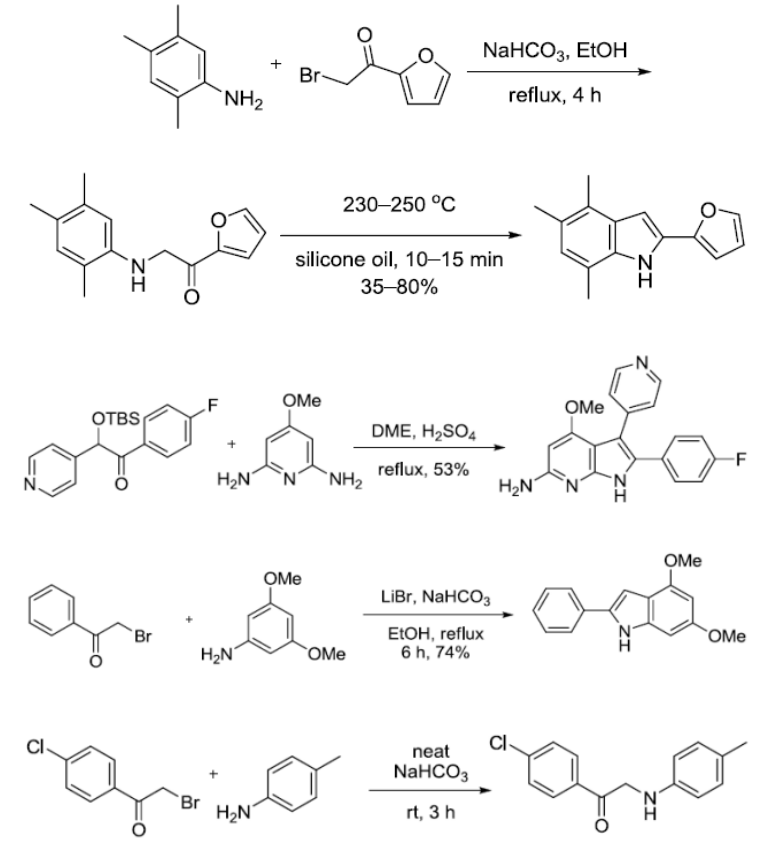
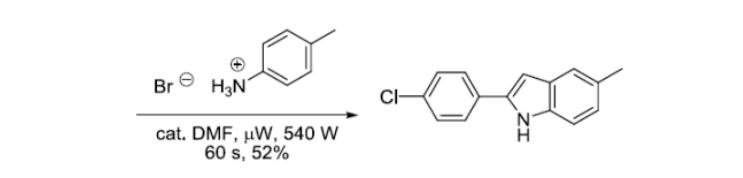
反应实例
参考文献
1. möhlau, r. ber. 1881,14, 171–175.
2. bischler, a.; fireman, p. ber.1893, 26, 1346–1349. augustus bischler (1865-1957)
3. sundberg, r. j. thechemistry of indoles; academic press: new york, 1970, pp 164.
4. buu-hoï, n. p.; saint-ruf,g.; deschamps, d.; bigot, p. j. chem. soc. (c) 1971,
2606–2609.
5. houlihan, w. j., ed.; thechemistry of heterocyclic compounds, indoles (part 1),
wiley: new york, 1972.(book).
6. bigot, p.; saint-ruf, g.;buu-hoï, n. p. j. chem. soc., perkin 1 1972, 2573–2576.
7. bancroft, k. c. c.; ward, t.j. j. chem. soc., perkin 1 1974, 1852–1858.
8. coïc, j. p.; saint-ruf, g.;brown, k. j. heterocycl. chem. 1978, 15, 1367–1371.
9. henry, j. r.; dodd, j. h. tetrahedronlett. 1998, 39, 8763–8764.
10. pchalek, k.; jones, a. w.;wekking, m. m. t.; black, d. s. c. tetrahedron 2005, 61,
77–82.
11. sridharan, v.; perumal, s.;avendaño, c.; menéndez, j. c. synlett 2006, 91–95.
12. zhang, j. bischler–möhlauindole synthesis, in name reactions in heterocyclic
chemistry ii; li, j. j., ed.; wiley: hoboken, nj, 2011, pp 84-90.(review).